Utsukushima (Beautiful Fukushima) Next-Generation Medical Industry Agglomeration Project > Project > Action Plans
Utsukushima (Beautiful Fukushima) Next-Generation Medical Industry Agglomeration Project > Project > Action Plans
We continue communicating with medical institutions and industries to pursue production of safe, high-quality and cost-saving medical devices.

(Pictures of conferences)
We provide business matching services to SMEs in Fukushima Prefecture to facilitate connections with medical device manufacturers and research institutions including universities, all located inside and/or outside the Prefecture.
We send project managers with outstanding skills and experiences in medical devices to regional SMEs to promote their business expansion to medical welfare related industry through various approaches: offer business matching services with medical device manufactures (prototype production, OEM production, component supply etc), introduce prototype production plans from university researchers, and support SMEs in raising research funds from third parties.
We pursue the development and commercialization of leading-edge diagnostic and therapeutic devices utilizing outcomes of research on haptic (tactile sensor) technology of the Nihon University College of Engineering as well as optical, miniaturization, and information-processing technologies.

We host seminars to encourage regional SMEs to collaborate with medical device manufactures and universities as well as reinforce skills required for device productions.

We encourage SMEs in non-medical sectors to enter into medical device related industries for the first time.
We offer SMEs independent consultation by sending them consultants with skills and experiences in medical devices.
We promote technological strengths of SMEs to the world in order to allow them to cultivate a marketing network in the following ways:

We financially support firms engaged in development and verification testing of medical and welfare devices, in order to aid in the recovery of the industries affected by the Tohoku Earthquake and Tsunami.
“Development of robotic suit HAL for medical usage“
The firm develops rehabilitation equipments for elbows and knees. The self-driven equipments aim to improve motor function of human beings including brain, nervous system and musculoskeletal systems.

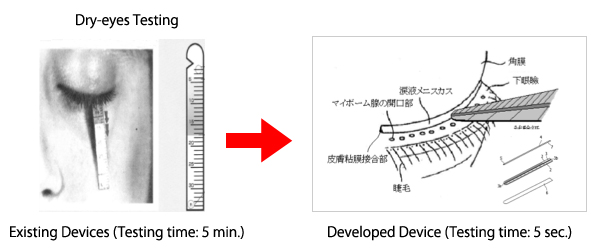
“Development of medical device for tear secretion examination”
The firm develops prompt and accurate dry-eyes testing (tear secretion examination) devices that shorten the regular testing time of 5 minutes to 5 seconds saving time for both patients and doctors.
“Development of magnetically guided capsule endoscope system for improving gastric cancer screening rate”
